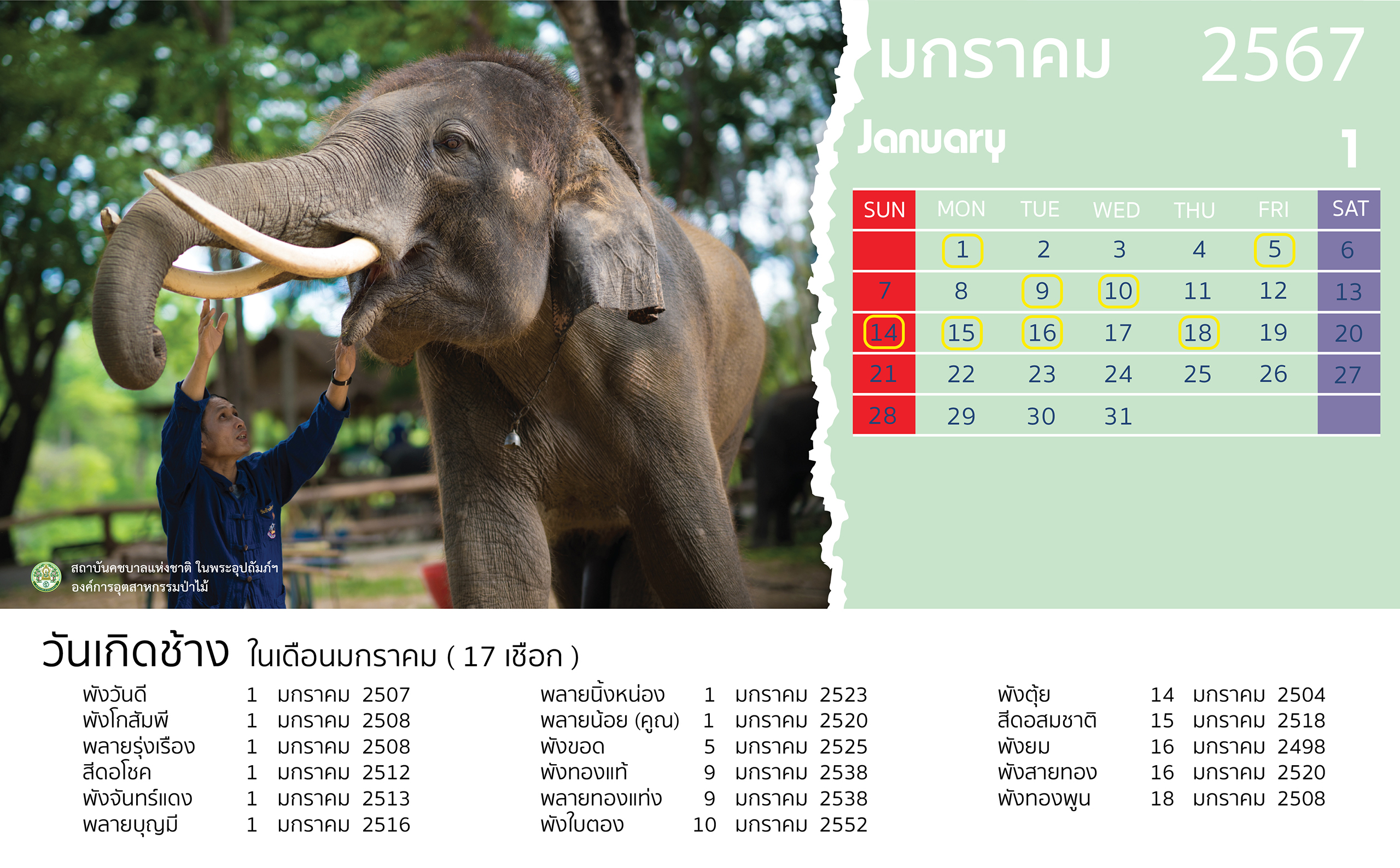
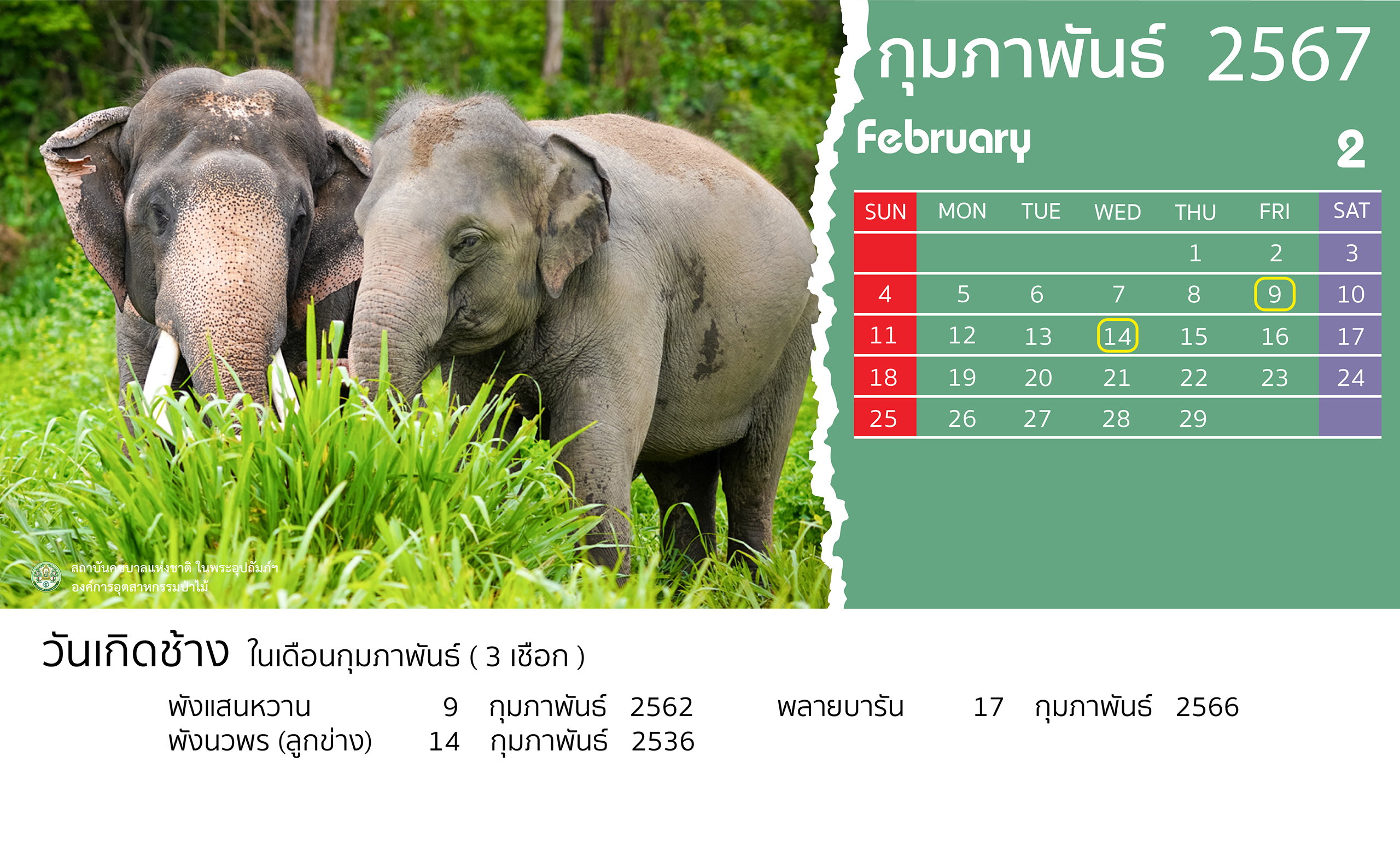
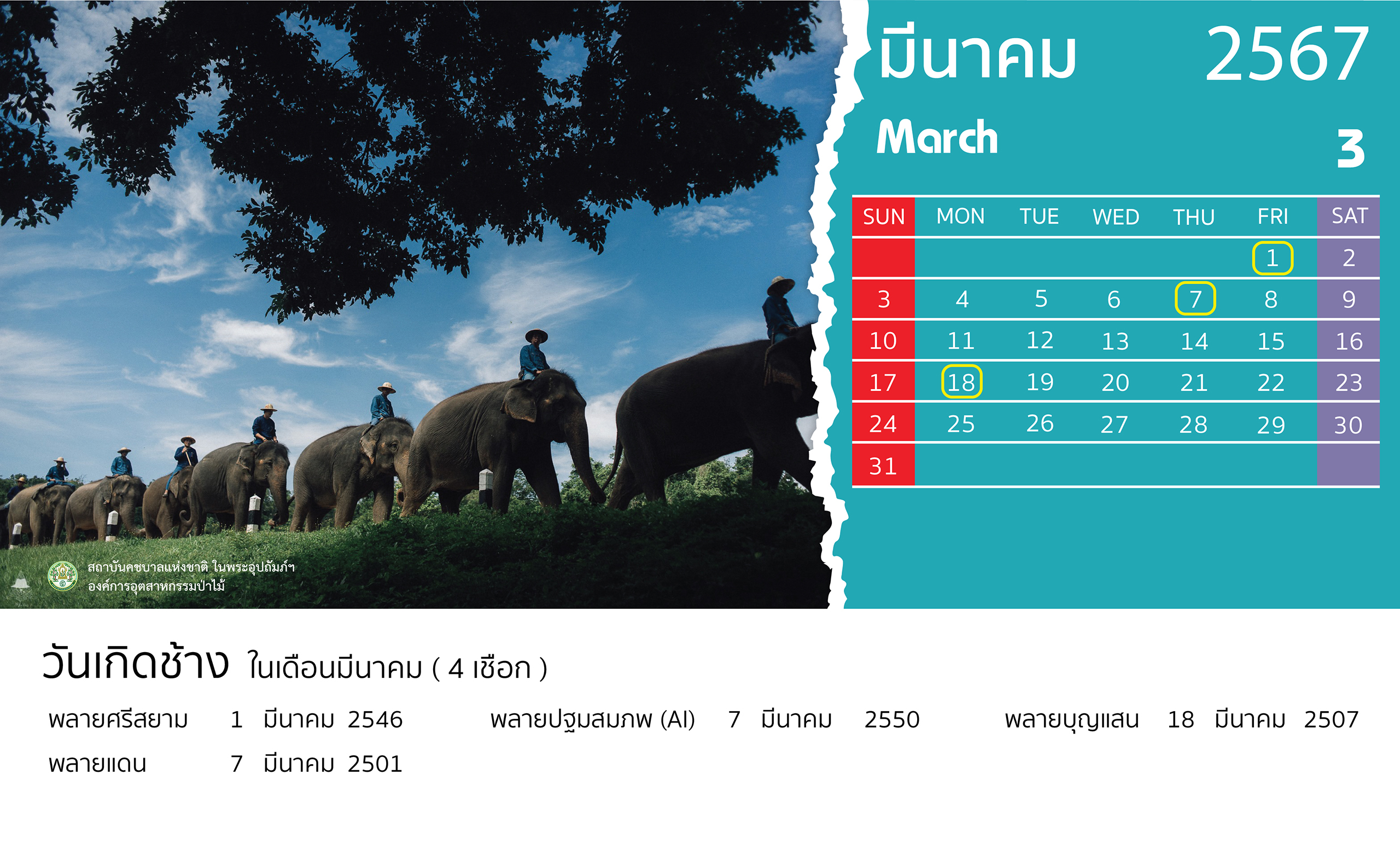
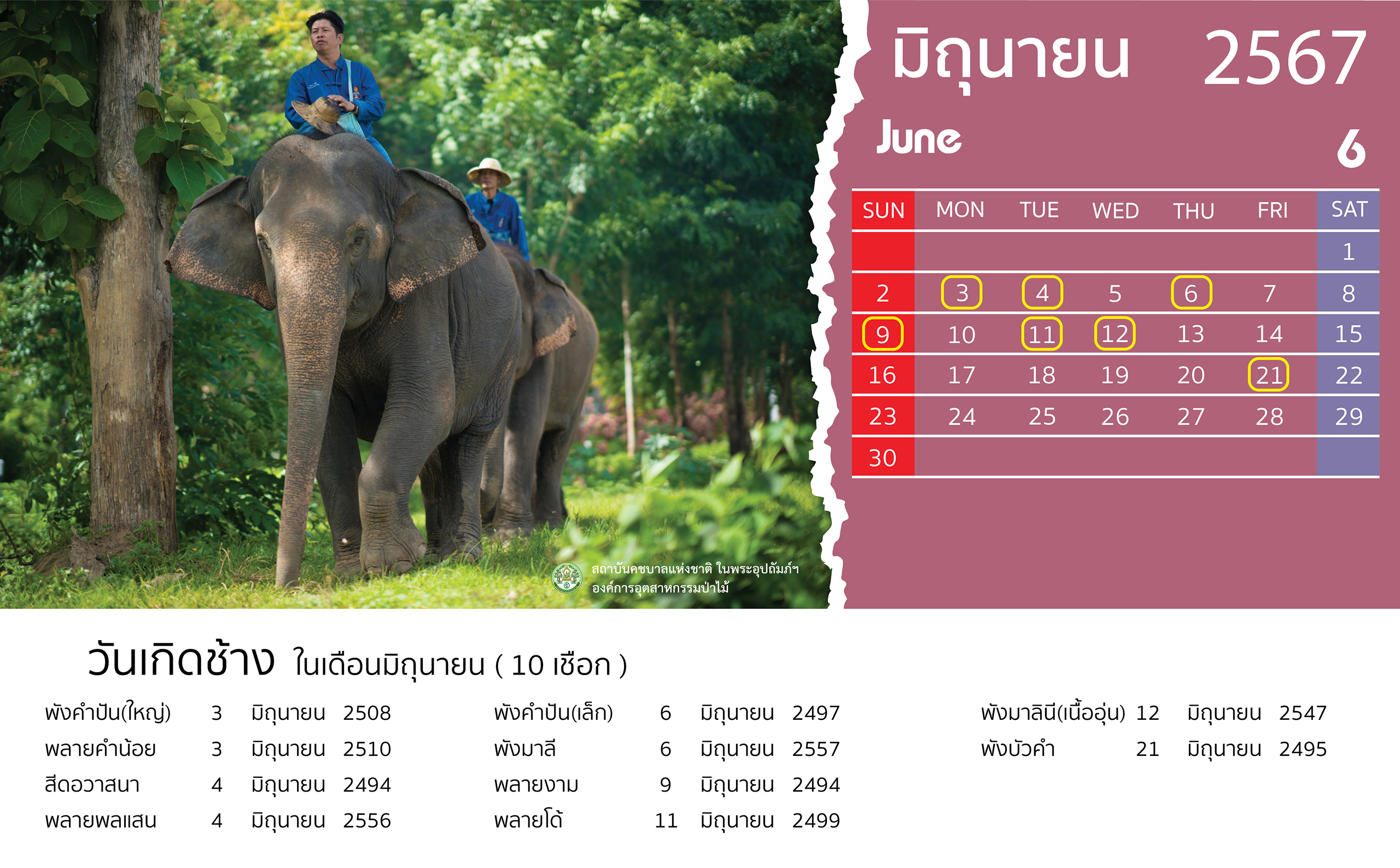
- 28 February 2024
ส.คช. ร่วมพิธีเจริญพระพุทธมนต์ถวายพระพรชัยมงคลฯ
- 17 January 2024
สถาบันคชบาลแห่งชาติ ในพระอุปถัมภ์ฯ รับมอบเงินบริจาค 1,000,000บาท
- 14 December 2023
บริษัทธนบดีเดคอร์เซรามิค จำกัด มอบเงินสนับสนุนช้างจำนวน 20,000 บาท
- 27 October 2023
มหกรรมดนตรีนานาชาติฮีนาสเตร่าและการท่องเที่ยวปี 2566
- 02 October 2023
ส.คช. ให้การต้อนรับคณะสัตวแพทย์จากรัฐเคเรล่า ประเทศอินเดีย
- 26 August 2023
ส.คช. ร่วมกับ มช. จัดการประชุมช้างแห่งชาติ ประจำปี 2566
- 27 June 2023
ส.คช. ร่วมถวายพระกุศลสมเด็จพระสังฆราชฯ
- 12 March 2024
ประกาศเผยแพร่แผนการจัดซื้อจัดจ้าง 2567 จำนวน 4 รายการ
- 20 October 2023
ประกาศเผยแพร่แผนการจัดซื้อจัดจ้าง 2 โครงการ
- 05 September 2023
ประกาศผู้ชนะการเสนอราคา ซื้อโซ่สำหรับควบคุมช้าง จำนวน 1 โครงการ (2
- 29 August 2023